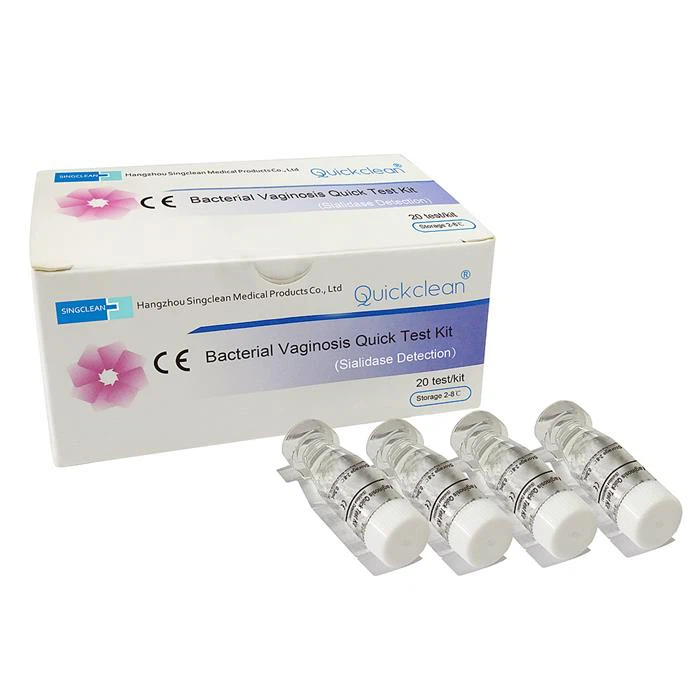
China Disposable Bacterial Vaginosis Rapid Diagnostic Test (dry chemical enzymatic detection), is an in vitro diagnostic reagentsproducts to test female bacterial vaginosis. Through testing the SNA, PH Value, LE, and H202, the four indicators of female vaginaldischarge, evaluate comprehensively, and get the result,it has certain guiding significance for the clinical diagnosis.
01
Senstivity
minimum detection level of neuraminidase should be 0.5 U/mL. Minimum detection level of leukocyte esterase shouldbe 0.3 U/mL. Minimum detection level of Hydrogen peroxide should be 2μmo/L.
02
Consistency
when a same reference is used to test 10 cases, the color results shall besame.
03
Specificity
it won't affect the result if hemoglobin concentration is lower than 50mg/dL.

For qualitative determining hydrogen peroxide (H2O2) concentration, pH value, neuraminidase (SNa) and leukocyte esterase (LE) activity in female vaginal secretion, only serving as assistant in diagnosis of bacterial vaginosis.
Specification
|
COMPONENTS |
DETAIL |
QUANTITY |
|
Individual packed BV test cassette |
Each device contains with treated membrane |
20pcs |
|
Extraction solution |
For specimen extraction |
12ml*1 bottle |
|
Chromogenic reagent |
For chromogenic reaction |
2ml*1 bottle |
|
Extraction tube |
For specimens preparation use |
20pcs |
|
Sterile Pipette |
For operating |
20pcs |
|
Package Insert |
For operation instruction |
1pc |
Store at 2°C ~ 8°C. It expires after 12 months. The product must be used within 8 hours after the aluminum foil bag for reaction strip is unpacked.

Complications And Prevention
Pelvic inflammatory disease
abnormal uterine bleeding and endometritis
gynecological infection after surgery
cervical cancer
HIV infection
infertility and abortion
amniotic chorioamnion, premature rupture of membranes, premature delivery and low birth weight infants
postpartum endometritis and cesarean section wound infection
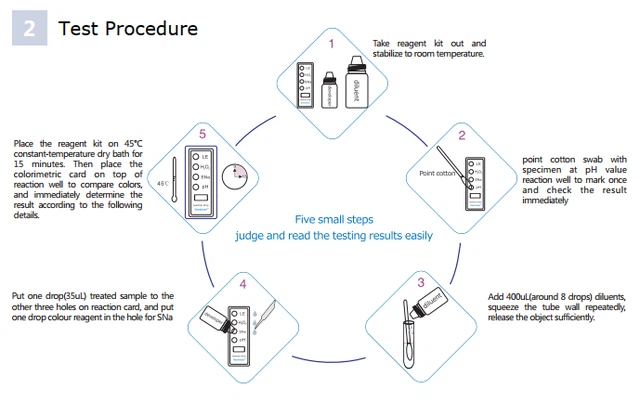
Test Procedure
·Read the entire procedure carefully prior to testing.
·Do not open the foil pouch until get ready to perform the test
1)Take out the test device from its sealed foil pouch
2)Swab the pH test well 2-3 times with swab which collects secretion specimen Observe the color change and record the pH value
3)lmmerse the patients swab into extraction tube and add 12-14 drops of extraction solution in it Squeeze the swab repeatedly toensure thorough extraction.
4)Add each 2 drops of extracted specimen from extraction tube to the rest three wells ( H2O2, SA, LE , PHvalue well)
5)Add a drop of chromogenic regent to the H2O2 test well
6)Place the test device into Encode Incubator or attemperat at 42~45°C or thermostat water bath.
7)Read the result within 15 mins
interpretation of results
(1) PH well: Yellow, greenish yellow or green color indicates normal result; the presence of greenish blue or blue indicates abnormal result.
(2) H2O2 well: Red indicates normal result; no color change indicates abnormal result
(3) SA well: Light blue or blue indicates positive result; no color change indicates negative result.
(4) LE well: Greenish blue or blue indicates positive result; no color change indicates negative result.
FAQ
Q: How does the Wholesale Women Bacterial Vaginosis Rapid Diagnostic Test involve?
Q: Why do I need this One Step Bacterial Vaginosis Rapid Diagnostic Test?
Q: Is the Home Rapid Bacterial Vaginosis Test similar to the doctor's test?
Q: How can you ensure the quality of your products?
Q: Does your factory do OEM?
Hot Tags: Quickclean Bacterial Vaginosis Combo Test Kit, China, manufacturers, suppliers, wholesale, hydraulic filler for face, injections for fine lines, non hcg pregnancy test, over the counter testosterone test kit, hyalone injection for knee, hyaluron pen cheek filler